Identification of new inhibitors of NS5 from dengue virus using saturation transfer difference (STD-NMR) and molecular docking studies†
Abstract
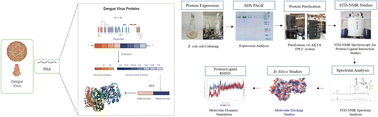
The rapid spread of dengue virus has now emerged as a major health problem worldwide, particularly in tropical and sub-tropical regions. Nearly half of the human population is at risk of getting infection. Among the proteomes of dengue virus, nonstructural protein NS5 is conserved across the genus Flavivirus. NS5 comprises methyltransferase enzyme (MTase) domain, which helps in viral RNA capping, and RNA-dependent RNA polymerase (RdRp) domain, which is important for the virus replication. Negative modulation of NS5 decreases its activity and associated functions. Despite recent advances, there is still an immense need for effective approaches toward drug discovery against dengue virus. Drug repurposing is an approach to identify the new therapeutic indications of already approved drugs, for the treatment of both common and rare diseases, and can potentially lower the cost, and time required for drug discovery and development. In this study, we evaluated 75 compounds (grouped into 15 mixtures), including 13 natural compounds and 62 drugs, by using biophysical methods, for their ability to interact with NS5 protein, which were further validated by molecular docking and simulation studies. Our current study led to the identification of 12 ligands, including both 9 US-FDA approved drugs and 3 natural products that need to be further studied as potential antiviral agents against dengue virus.
- This article is part of the themed collection: 2022 RSC Advances Popular Advances Collection


 Please wait while we load your content...
Please wait while we load your content...