Acylmonofluoromethylation of alkenes via dual NHC/photoredox catalysis†
Abstract
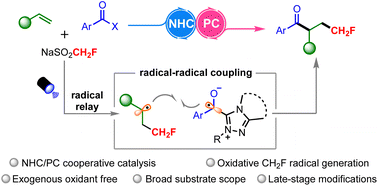
Monofluoromethylated compounds exhibit unique physicochemical properties with wide applications in drug discovery. The direct monofluoromethylation of common feedstocks such as alkenes is an attractive approach. Herein, we report a novel three-component radical acylmonofluoromethylation of alkenes by cooperative NHC/photoredox catalysis to synthesize various monofluorinated alkyl aryl ketones. This protocol proceeds through radical–radical coupling of ketyl radicals with benzylic C-radicals. This reaction features broad substrate scope, excellent functional group tolerance and mild conditions. Also, facile late-stage modification of drug molecules and natural products is demonstrated. The SET oxidative generation of monofluoromethyl radicals is advantageous over the use of excess exogenous oxidants, and may open up new avenues for the preparation of monofluorinated building blocks.


 Please wait while we load your content...
Please wait while we load your content...