Regio- and stereoselective divergent cross-coupling of alkynes and disubstituted alkenes via photoredox cobalt dual catalysis†
Abstract
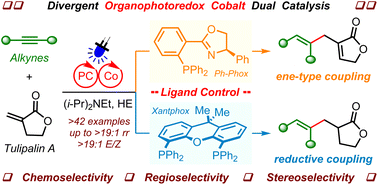
Achieving divergent synthesis from the same substrates quickly to generate different products remains an attractive objective in synthetic chemistry and the medicinal industry. Here, we report a discovery of highly selective divergent cross-coupling of alkynes and disubstituted alkenes by merging visible light photoredox and cobalt catalysis. Under otherwise identical conditions, the use of either a hemilabile P,N-ligand or a strong bidentate diphosphine ligand leads to ene-type coupling or reductive coupling of alkynes and Tulipalin A, producing stereodefined 1,4-diene or trisubstituted alkene products, respectively. The approaches feature considerable advantages for the straightforward synthesis of stereodefined multiple substituted alkenes, such as easily available substrates, low catalyst loading of an organophotocatalyst, excellent regio- and stereoselectivity, good functional group tolerance, and mild reaction conditions. Reasonable catalytic reaction pathways from the same cobaltacyclopentene intermediate have been proposed.


 Please wait while we load your content...
Please wait while we load your content...