One-pot and sustainable liquid-phase peptide extension for synthesis of C-terminal amidation peptides aided by small molecular tags†
Abstract
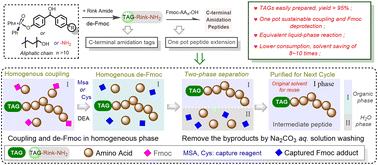
The development of greener and more economical methods for peptide synthesis has been a driving force in promoting the large-scale production of peptide drugs. In this study, we present a series of small-molecule C-terminal amidation tags based on phosphonate or aliphatic moieties. These tags were prepared using a simple method with high yields and employed to facilitate the sustainable preparation of C-terminal amidation peptides via an Fmoc strategy liquid-phase one-pot process. The Fmoc group was deprotected using DEA/ACN and subsequently reacted with mercaptosuccinic acid or cysteine to capture and remove the Fmoc-residue. In the continuous one-pot peptide chain coupling and de-Fmoc reactions, each peptide intermediate can be easily purified through sequential washing with HCl aq. and Na2CO3 aq. solutions, allowing subsequent amino acid couplings to proceed in the original reaction solvent. Using the one-pot peptide synthesis strategy, both eptifibatide and the TREM-1 inhibitor LR12 were efficiently synthesized. Compared to LPPS and SPPS, this protocol achieves a more environmentally friendly and cost-effective method for preparing amidated peptides, making it suitable for large-scale production of peptide APIs.


 Please wait while we load your content...
Please wait while we load your content...