N-Heterocyclic carbene catalyzed synthesis of benzotrifluorides from enals and β-trifluoromethylenones†
Abstract
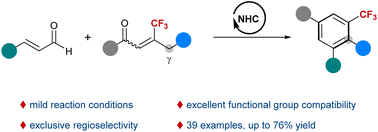
An NHC-catalyzed benzannulation reaction of enals and β-trifluoromethylenones is developed for the synthesis of benzotrifluorides. This process involves an NHC-catalyzed [4 + 2] annulation/lactonization/decarboxylation/oxidative aromatization cascade. The reaction features mild reaction conditions, excellent functional group compatibility, and exclusive regioselectivity. A series of multi-substituted benzotrifluorides were obtained in moderate to good yields.
- This article is part of the themed collection: 2023 Organic Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...