Nitro – a traceless directing group for reversing the radical site-selectivity of styrene derivatives†
Abstract
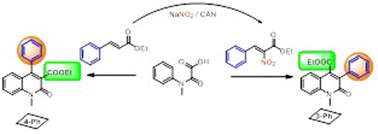
2-Quinolone is a privileged nitrogen heterocycle that is found in various bioactive and functional substances. Herein, a two-component [4 + 2] annulation reaction of styrene derivatives with aryloxalamides via a decarboxylative radical process is proposed, which produced two series of position-switched products depending on a nitro or non-nitro directed process. Our work should be the first attempt to use nitro as a traceless directing group for reversing the site-selectivity of styrene, through which 4-phenyl-2-quinolone (4-Ph) or 3-phenyl-2-quinolone (3-Ph) could be obtained selectively. Density functional theory (DFT) calculations suggested that the reactions undergo a radical pathway via decarboxylation, cycloaddition and rearomatization processes.


 Please wait while we load your content...
Please wait while we load your content...