Asymmetric catalytic Friedel–Crafts alkylation with arenes and heteroarenes: construction of 3,3-disubstituted oxindoles†
Abstract
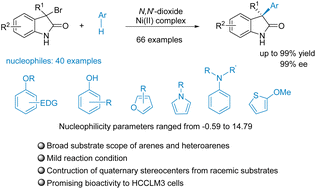
The asymmetric synthesis of enantioenriched C3-arylated oxindoles bearing a tetrasubstituted stereocenter has been achieved using a chiral N,N′-dioxide/transition metal complex which promoted the Friedel–Crafts alkylation of 3-bromo-3-substituted oxindoles with arenes and heteroarenes, i.e., anisole derivatives, furans, pyrroles, 2-methoxythiophene, anilines, and phenols. A series of 3-aryl-indolinones with quaternary stereocenters have been obtained in up to 99% yields and 99% ee. In addition, the rationale for the reactivity is based on the nucleophilicity parameters of arenes and heteroarenes, which were predicted from Mayr's Database. Also, a gram-scale synthesis has been achieved and this method has been applied to the synthesis of bioactive compounds. Additionally, a plausible catalytic cycle has been proposed.


 Please wait while we load your content...
Please wait while we load your content...