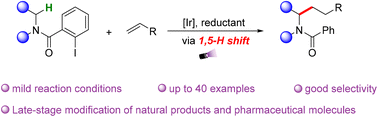
Selective N-α-C–H alkylation of cyclic tertiary amides via visible-light-mediated 1,5-hydrogen atom transfer†
Abstract
Herein, we report an effective C–H bond activation-alkylation strategy for 2-iodobenzoyl protected cyclic amines at the N-α-position through a visible-light mediated 1,5-hydrogen atom transfer (HAT) process. The readily reducible CAr–I bond was selected as an appropriate precursor to generate aryl radicals, which would further trigger an intramolecular HAT process to access the key N-α-radicals. Scale-up reaction and several examples of late-stage functionalization on structurally complex bio-active molecules demonstrate the synthetic potential of this protocol.


 Please wait while we load your content...
Please wait while we load your content...