Investigation of the activation mode of vinylarene in Giri's Ni-catalyzed vinylarene intermolecular 1,2-alkylarylation cross-coupling: a theoretical study†
Abstract
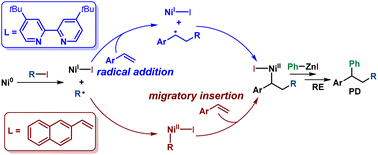
Ni-catalyzed alkene intermolecular 1,2-dicarbofunctionalization cross-coupling is an efficient method to construct complex molecules from readily available starting materials. Typically, alkenes are proposed to be activated by a radical addition mode in this cross-coupling reaction. However, in this report, we present computational evidence that supports a migratory insertion activation mode of alkenes in the Ni-catalyzed vinylarene 1,2-alkylarylation cross-coupling reaction. The ligand effect was investigated using bipyridyl as a ligand in the 1,2-alkylarylation cross-coupling reaction. When bipyridyl was used as a ligand, the activation mode of vinylarene was radical addition rather than migratory insertion because of the high activation barrier of the migratory insertion transition state. Global electrophilicity index and global nucleophilicity index calculations were conducted to elucidate the cause for the selection of the migratory insertion activation mode by vinylarene. Moreover, the selectivity between two-component and three-component cross-coupling was also investigated. This study will provide a theoretical guide for further alkene 1,2-dicarbofunctionalization cross-coupling experimental investigations.


 Please wait while we load your content...
Please wait while we load your content...