TBAF-promoted carbanion-mediated sulfonamide cyclization of CF3-substituted N-allenamides: an access to fluorinated γ-sultams†
Abstract
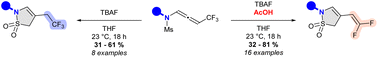
Upon treatment with TBAF, CF3-substituted N-allenamides were transformed into γ-sultams. Cyclic sulfonamides bearing an ene-gem-difluorinated tether could be obtained by addition of acetic acid to the ammonium salt whereas TBAF alone provided the corresponding trifluorinated ethyl sultams. A combined experimental and computationnal mechanistic study suggested that this transformation involves a 5-endo-dig cyclization on the ene-ynamide generated in situ.


 Please wait while we load your content...
Please wait while we load your content...