Direct benzylic polychlorination of (poly)azines with N-chlorosuccinimide†
Abstract
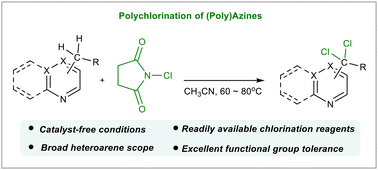
Herein, we describe a site-selective benzylic C–H bond chlorination of nitrogen-containing heteroarenes using low-cost N-chlorosuccinimide in acetonitrile under catalyst- or initiator-free conditions. This protocol is applicable to a wide range of N-heterocycles with excellent chemoselectivity, affording benzylic di- and/or trichlorinated azines under mild conditions. The practicality of this protocol was demonstrated by a scale-up experiment. Besides, both the chlorinated product and the by-product succinimide are readily isolated via a column chromatography-free purification process, making this method efficient and sustainable.


 Please wait while we load your content...
Please wait while we load your content...