Asymmetric binary-acid catalysis: a diastereo- and enantioselective oxa-Nazarov cyclization-Michael addition of conjugated 1,2-diketones†
Abstract
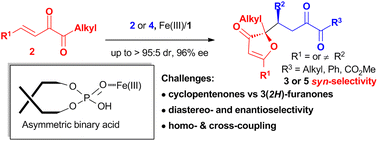
Diastereo- and enantioselective oxa-Nazarov cyclization-Michael addition of conjugated 1,2-diketones was achieved by using asymmetric binary-acid catalysis with chiral phosphoric acid and FeBr3. The reaction affords the corresponding chiral 3(2H)-furanones in good yields and with high stereoselectivity (up to >95 : 5 dr and 96% ee).


 Please wait while we load your content...
Please wait while we load your content...