An alternative metal-free amination approach to 3-trifluoromethyl aniline derivatives: the major products under Kröhnke pyridine synthesis conditions†
Abstract
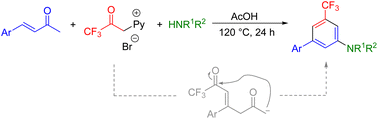
As an alternative metal-free amination, we report a simple and efficient annulation reaction of 1-(3,3,3-trifluoro-2-oxopropyl)pyridin-1-ium bromide and α,β-unsaturated carbonyl compounds with NH4OAc or amines. With the developed protocol, a series of 3-trifluoromethyl aniline derivatives as the major products were obtained in good to excellent yields under Kröhnke pyridine synthesis conditions. The reaction proceeds via a cascade process involving the 1,4-Michael addition of the pyridinium ylide to an α,β-unsaturated carbonyl compound, followed by intramolecular addition of a carbanion to the keto carbonyl group to form a dienone intermediate.


 Please wait while we load your content...
Please wait while we load your content...