Iron-catalyzed cross-electrophile coupling of bromostyrenes and chlorosilanes†
Abstract
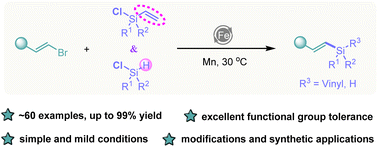
We report here a facile and efficient vinylation method using Fe-catalyzed cross-electrophile coupling of readily available vinyl- and hydro-chlorosilanes with a variety of substituted alkenyl bromides using manganese as the terminal reductant. This C(sp2)–Si forming modular approach shows excellent functional group tolerance and broad substrate scope, which allows the creation of a series of vinyl organosilanes, including electron-rich, electron-poor, and ortho-/meta-/para-substituted vinyl electrophiles, which were coupled successfully. Moreover, several substrates with structurally complex natural products and pharmaceutical motifs were well modified by this vinyl silylation process. Gram-scale reaction and derivatization of the formed vinyl organosilanes are demonstrated.


 Please wait while we load your content...
Please wait while we load your content...