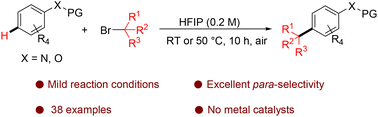
HFIP-promoted para-selective alkylation of anilines and phenols with tertiary alkyl bromides†
Abstract
A hexafluoroisopropanol-promoted highly regioselective Friedel–Crafts alkylation of arenes has been developed in this study. Various arenes, including anilides, anisoles, and phenols, were compatible with the reaction, leading to para-alkylated products in moderate to excellent yields. Preliminary mechanistic studies revealed that HFIP acted as a weak Lewis acid to activate haloalkanes and coordinated with arenes through hydrogen bonding to increase the steric hindrance of the coordinated functional group, affording a highly para-selective alkylated product.


 Please wait while we load your content...
Please wait while we load your content...