Cooperative photocatalysis and l-/d-proline catalysis enables enantioselective oxidative cross-dehydrogenative coupling of acyclic benzylic secondary amines with ketones†
Abstract
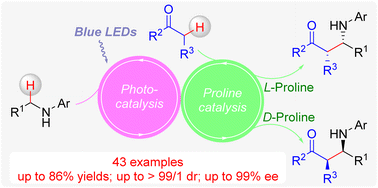
Herein, we developed a new enantioselective oxidative cross-dehydrogenative coupling of acyclic benzylic secondary amines with simple ketones by combining photocatalysis and L-/D-proline catalysis. A variety of acyclic benzylic secondary amines were transformed into the corresponding β-amino carbonyl compounds in good yields up to 86% with high enantio- and diastereoselectivities (up to 99% ee and > 99 : 1 dr) under mild and oxidant-free conditions. This novel protocol has characteristics of wide substrate scope, excellent functional group tolerance, and simple operation. In addition, the desired products with opposite configurations can be easily obtained by employing cheap and commercially available L-/D-proline as chiral organocatalysts.


 Please wait while we load your content...
Please wait while we load your content...