HTE and machine learning-assisted development of iridium(i)-catalyzed selective O–H bond insertion reactions toward carboxymethyl ketones†
Abstract
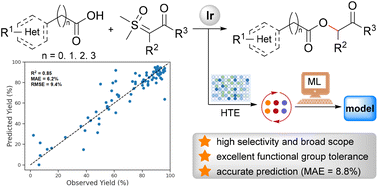
Functional group substituted carboxymethyl ketones, particularly heterocyclic ones, are important structural motifs in biologically active molecules, but their synthesis is challenging. In this work, by combining HTE and machine learning technologies, an iridium(I)-catalyzed highly selective O–H bond insertion reaction of carboxylic acids and sulfoxonium ylides is developed, which is compatible with amine, phenol, alcohol, and free indole functional groups, and efficiently produces various (hetero)carboxymethyl ketones. Moreover, an extensive reaction space exploration is accomplished. Based on the pre-trained PanGu-Drug-Model, we have built a type-specific reaction yield prediction model which shows excellent performance (mean absolute error in yield is 8.8% in an external test), demonstrating the application potential of the model. A web server implemented using the fine-tuned PanGu-Drug-Model is available via the internet at https://www.pangu-drug.com/ylide.
- This article is part of the themed collection: 2023 Organic Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...