Cyclopropanation of N-vinylimides via a redox-neutral photocatalysed radical addition/anionic cyclisation process†‡
Abstract
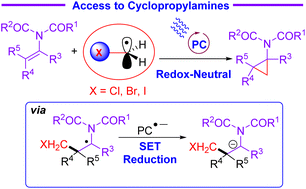
The radical addition/SET reduction/3-exo-tet cyclisation process has emerged as a useful and powerful strategy for the preparation of functionalized cyclopropanes. Herein, a novel protocol dealing with the preparation of cyclopropylamines has been successfully developed via the reaction of N-vinylimides with halomethylsilicate enabled by mild and redox-neutral photocatalysis. A mechanism based on a reductive radical–polar crossover process has been proposed. The stereoconvergence of this cyclopropanation reaction of N-vinylimides has been demonstrated. Moreover, synthetic applications of imido-substituted cyclopropanes have also been highlighted by the deprotection of protective groups.


 Please wait while we load your content...
Please wait while we load your content...