Cu-catalyzed reductive aminomethylation of 1,3-dienes with N,O-acetals: facile construction of β-chiral amines with quaternary stereocenters†
Abstract
A copper-catalyzed highly regio- and enantioselective reductive aminomethylation of 1,1-disubstituted 1,3-dienes with N,O-acetals is reported for the first time. This work provides expedient access to β-chiral amines with all-carbon quaternary stereocenters, motifs commonly found in pharmaceuticals and natural products. Moreover, this method features good functional group tolerance, facile scalability, and easy derivatization of products to useful building blocks.
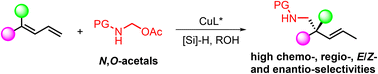


 Please wait while we load your content...
Please wait while we load your content...