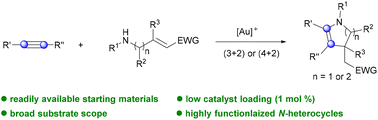
Gold-catalyzed formal (3 + 2) and (4 + 2) cycloadditions of alkynes to highly functionalized dihydropyrroles and tetrahydropyridines†
Abstract
Herein, we report a gold-catalyzed formal cycloaddition reaction of readily available alkynes using amino but/pent-2-enoate derivatives as annulating reagents. The new strategy provides a straightforward and convergent route towards the synthesis of highly functionalized 2,3-dihydropyrroles and 1,2,3,4-tetrahydropyridines with high efficiency and a broad substrate scope. Moreover, the reaction could be facilely performed at the millimole scale and the products could be further transformed into other valuable classes of N-heterocycles.


 Please wait while we load your content...
Please wait while we load your content...