Tunable synthesis of α,β-multifunctionalized azaheterocycles via the cascade reaction of saturated cyclic amines with diverse nucleophiles promoted by oxoammonium salt†
Abstract
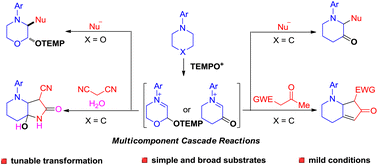
A convenient protocol for the synthesis of α-substituted-β-oxo azaheterocycles through the oxoammonium salt-promoted cascade reaction of saturated cyclic amines with different nucleophiles based on β-oxo cyclic iminium ion intermediates has been developed. Interestingly, when active methylene compounds containing the acetyl or cyano group were used as nucleophiles, piperidocyclopent-2-enones or 7-hydroxypyrrolo[3,2-b]piperidine-2-ones could be respectively obtained via a further intramolecular nucleophilic addition of the in situ generated α-alkyl-β-oxo azaheterocycles. In addition, the reaction of morpholines with nucleophiles under similar conditions furnished α-substituted-β-TEMPO decorated amines via β-TEMPO-cyclic iminium ion intermediates. Experimental studies and DFT calculations unveiled the origin of the chemoselectivity in the formation of different intermediates.


 Please wait while we load your content...
Please wait while we load your content...