Copper-catalyzed enantioselective fluoroalkenylation of cyclic imino esters†
Abstract
A copper-catalyzed enantioselective fluoroalkenylation of cyclic imino esters was developed. Under mild conditions, fluoroalkenylated products of cyclic imino esters were delivered in good yields with a broad substrate scope. A full-substituted carbon center along with a tetrasubstituted fluoroalkene was forged with good stereoselectivity. Late-stage functionalization of pharmaceuticals was also illustrated through this protocol with high enantioselectivities.
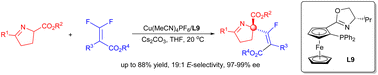


 Please wait while we load your content...
Please wait while we load your content...