Polymers from sugars and isothiocyanates: ring-opening copolymerization of a d-xylose anhydrosugar oxetane†
Abstract
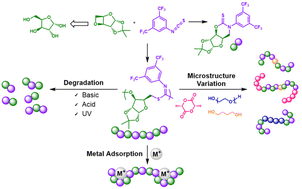
A D-xylose 3,5-anyhydrosugar (D-Ox) has been applied in the ring-opening copolymerisation (ROCOP) with isothiocyanates to form alternating AB-type copolymers with imidothiocarbonate linkages (Mn ≥ 34 900 g mol−1). Catalysed by an Al(III)-based aminotrisphenolate complex, ROCOP proceeds with high selectivity with four aromatic isothiocyanates, including di-isothiocyanates used for crosslinking, to form thermally robust polymers (Td,5% >228 °C) with a range of high glass-transition temperatures (76–134 °C). Kinetic studies show a reaction order of two with respect to the binary catalytic system, and a first order with respect to D-Ox. When using an Al(III) porphyrin complex as catalyst, the reactivity can be tailored to form exclusively a non-polymerisable cyclic thionocarbamate byproduct. The topology, thermal and physical properties of the polymer could be altered by adding a difunctional isothiocyanate crosslinker. The synthesis of di and triblock copolymers was possible by using difunctional (macro)initiators and by exploiting the living character of the ROCOP process, which allowed chain-extension by the ring-opening polymerisation (ROP) of lactide. The polymers readily degrade under acidic and basic conditions. Photodegradation without additives is also possible with an 85% decrease in molar mass in one week. The potential of the imidothiocarbonate linkages for metal capture has been investigated, and good affinity for Cu2+ ions is seen.


 Please wait while we load your content...
Please wait while we load your content...