Isoquinoline-1,3-dione-derived conjugated polymers for field-effect transistors: synthesis, properties, and the effect of inner aromatic bridges†
Abstract
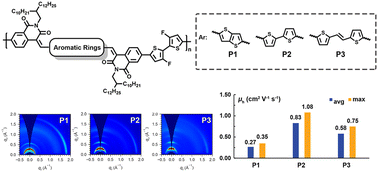
Isoquinoline-1,3-dione (IQD) is a novel electron-withdrawing building block. However, its polymer derivatives have been rarely constructed and studied. Herein, we report a series of polymers (P1–P3) based on three novel IQD-derived electron-acceptors 5a–c, which were synthesized via aldol condensation reactions between IQD and the dialdehyde of thieno[3,2-b]thiophene (TT), 2,2′-bithiophene (BT), and (E)-2-(2-(thiophen-2-yl)vinyl)thiophene (TVT), respectively. P1-based field-effect transistors showed ambipolar charge transport behavior with the electron/hole mobilities (μe/μh) of 0.04/0.35 cm2 V−1 s−1. However, P2- and P3-based ones showed p-type behaviors with high μh values of 1.08 and 0.75 cm2 V−1 s−1, respectively, which are among the highest in the IQD-based polymers reported so far. Thin film microstructural analyses demonstrated that the more extended π-conjugation and less backbone rigidity of bridging units, and better long-range order in thin films are responsible for the high μh of P2 and P3. Our work highlights that IQD is a promising building block for achieving high-performance semiconducting materials and its inner bridging units also have an important influence on the charge transport properties of polymer derivatives.


 Please wait while we load your content...
Please wait while we load your content...