Diversity-oriented synthesis of chemically recyclable poly(sulfonamide ester)s through organocatalytic aziridine-based multicomponent polymerization†
Abstract
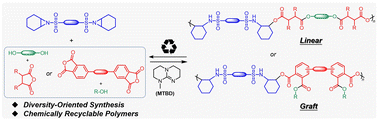
Aziridine is a highly reactive strained ring that allows the synthesis of diverse small molecules. However, few studies have been reported to utilize aziridine to achieve the structural diversity of polymers. Herein, we report an aziridine-based multicomponent polymerization approach toward building-block diversity and architectural diversity. Bis(N-sulfonyl aziridine) polymerizes with diol and anhydride efficiently in the presence of 7-methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene (MTBD), producing linear poly(sulfonamide ester)s with diverse ester building blocks. Graft poly(sulfonamide ester)s are facilely synthesized using bis(anhydride) and alcohol as the monomers. Different side chains have been incorporated into the polymers, including terminal alkyne, hydrophobic fluoroalkane, and hydrophilic polyethylene glycol. The carboxylic ester groups of poly(sulfonamide ester)s can be hydrolyzed under alkaline conditions to give bis(sulfonamide alcohol), which readily converts to the bis(N-sulfonyl aziridine) monomer by two-step reactions. The synthesized polymers are thermally stable with decomposition temperature at 5% weight loss (Td,5%) up to 336 °C. This aziridine-based multicomponent polymerization approach is anticipated to enrich the library of aziridine-based polymeric materials and promote the development of diversity-oriented polymerization.


 Please wait while we load your content...
Please wait while we load your content...