Meldrum's acid mediated ketene chemistry in the formation of ester bonds for the synthesis of vitrimers with high glass transition temperatures†
Abstract
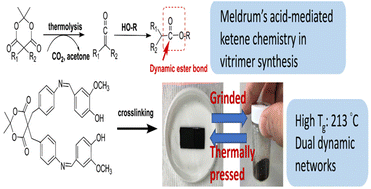
Dynamic ester bonds are widely employed to build covalent adaptable networks (CANs) and prepare the corresponding vitrimers. Conventional carboxylic anhydride/acid–epoxy chemistry facilitates the formation of dynamic ester bonds and generation of catalytic –OH groups; however, it provides low flexibility for molecular designs and property adjustments pertaining to vitrimers. This study demonstrates Meldrum's acid-mediated ketene chemistry as an effective method for building CANs and preparing the corresponding vitrimers. Through the employment of a newly synthesized AB2-type (A, Meldrum's acid group; B, phenol group) monomer (MADV), crosslinked MADV (CR-MADV) with dynamic ester linkages and free –OH groups is obtained through MADV self-polymerization. The prepared CR-MADV exhibits not only features of vitrimers but also favorable properties comparable to those of conventional thermosetting resins, including a thermal stability of 345 °C, a char yield of 53 wt% at 800 °C, a storage modulus of 3.2 GPa at 50 °C, and a Tg of 213 °C (observed from the tan δ peak during dynamic mechanical analysis). The prepared CR-MADV vitrimer has a higher Tg compared to those reported in other studies and still exhibits mendable and recycling features. This study contributes substantially to the development of synthetic methods and the enhancement of vitrimer properties.


 Please wait while we load your content...
Please wait while we load your content...