Influences of nitrogen base excess on ARGET ATRP of styrene with ascorbic acid acetonide and traces of oxygen and water†
Abstract
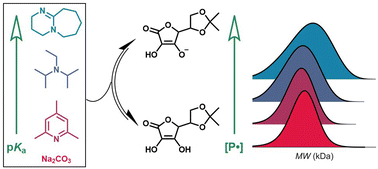
Ascorbic acid is a promising regenerating agent for Activators ReGenerated by Electron Transfer Atom Transfer Radical Polymerization (ARGET ATRP) thanks to its sustainability and environmental friendlyness. The ascorbate anion has even more potential because it has a higher kinetic rate constant of reduction toward the copper catalyst than its protonated counterpart. Although ascorbic acid can be easily neutralized with inorganic bases, the resulting heterogeneous system in the polymerization of hydrophobic monomers (such as styrene) is not well-suited for industrial applications. To overcome this problem, in this study we investigate the use of ascorbic acid acetonide, a more lipophilic derivative, together with soluble nitrogen bases of different basicity. The results show how the pKa of the protonated form of the nitrogen base affects the process, especially in the presence of traces of water and/or oxygen. Additionally, we report that milder bases yield better results in terms of dispersity and chain-end fidelity, while high pKa bases lead to a complete loss of control.


 Please wait while we load your content...
Please wait while we load your content...