A novel polymer electrolyte with in situ polymerization and a high concentration of lithium salts for lithium metal batteries†
Abstract
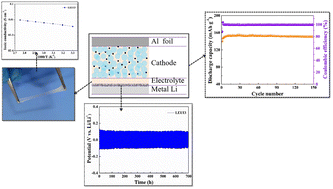
A novel polymer electrolyte is prepared by in situ polymerization for lithium metal batteries. The polymer electrolyte with in situ polymerization can maintain good inter-facial contact property between the electrodes and electrolyte. It is proved that the interface contact between the Li metal and the polymer electrolyte is very good. And the electrolyte is joined to the cathode electrode by in situ polymerization. As a result, the inter-facial resistance of the electrodes and electrolyte is small. Herein, the polymer electrolyte is amorphous. Thus, the novel polymer electrolyte has a high ionic conductivity (3.4 × 10−3 S cm−1) at room temperature and an electrochemical stability window (5 V, vs. Li/Li+). Furthermore, the polymer electrolyte is stable on the lithium metal anode. The polymer electrolyte also has good mechanical properties and can adapt to the changes of material volume and stress during charge and discharge. Thus, it can maintain the interface stability. As a result, the LiFePO4/polymer electrolyte/Li battery has good cycling stability and rate performance.


 Please wait while we load your content...
Please wait while we load your content...