Construction of diverse spirooxindoles via a domino reaction of arylamines, but-2-ynedioates and 3-hydroxy-3-(indol-3-yl)indolin-2-ones†
Abstract
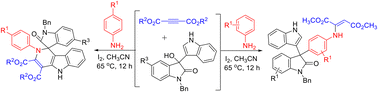
An iodine-promoted domino reaction of arylamines/benzylamines, dialkyl but-2-ynedioates and 3-hydroxy-3-(indol-3-yl)indolin-2-ones showed very interesting molecular diversity. The reaction in acetonitrile at 65 °C in the presence of 30% mmol I2 resulted in spiro[indoline-3,1′-pyrido[4,3-b]indoles] in satisfactory yields. When anilines without para-substituents were used in the reaction, a direct substitution of the hydroxyl group to 2-(phenylamino)maleate at the para-position of aniline gave chain products in good yields. Additionally, similar reactions with benzylamines not only gave spiro[indoline-3,1′-pyrido[4,3-b]indoles], but also afforded spiro[indoline-3,1′-pyrano[4,3-b]indol]-2-ones in lower yields. A plausible domino annulation mechanism was rationally proposed for the formation of different kinds of polycyclic compounds.


 Please wait while we load your content...
Please wait while we load your content...