Palladium-catalyzed decarboxylative α-allylation of thiazolidinones and azlactones with sulfonamido-substituted acyclic allylic carbonates†
Abstract
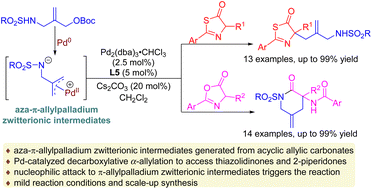
A palladium-catalyzed decarboxylative α-allylation of thiazolidinones and azlactones with aza-π-allylpalladium zwitterionic intermediates, in situ generated from sulfonamido-substituted allylic carbonates, is successfully developed. This method allows the formation of a series of structurally diverse 5-alkylated thiazolidinones and 2-piperidones under mild conditions in moderate to high yields (up to 99% yield).


 Please wait while we load your content...
Please wait while we load your content...