Macrocyclization strategies for the total synthesis of cyclic depsipeptides
Abstract
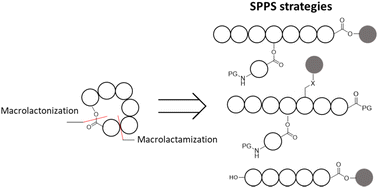
Cyclic depsipeptides are an important class of peptide natural products that are defined by the presence of ester and amide bonds within the macrocycle. The structural diversity of depsipeptides has required the development of a broad range of synthetic strategies to access these biologically active compounds. Solid phase peptide synthesis (SPPS) strategies have been an invaluable tool in their synthesis. The key aspect of their synthesis is the macrocyclization strategy. Three main strategies are used, solution phase macrolactamization of acyclic ester containing peptide, on-resin macrolactamization of a sidechain-anchored peptide, and the solution phase macrolactonization of a linear peptide. Additionally, biocatalysts have been used to produce these compounds in a regio- and chemo-selective manner. Each compound offers unique challenges, requiring careful synthetic design to avoid undesirable side reactivity or unwanted epimerization during the esterification and macrocyclizing steps. This focused review analyzes these three strategies for cyclic depsipeptide natural product total synthesis with selected examples from the literature between 2001–2023.


 Please wait while we load your content...
Please wait while we load your content...