Divergent transformation of C,N-cyclic-N′-acyl azomethine imines by reaction with diazo compounds†
Abstract
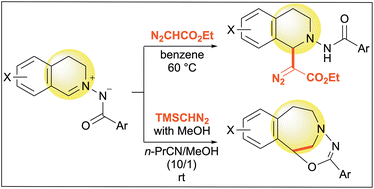
C,N-cyclic-N′-acyl azomethine imines with isoquinoline skeletons were investigated for their reactivity with diazo compounds via two different pathways. During the reaction with ethyl diazoacetate, an α-diazoacetate moiety was introduced at the C1-position of the resulting tetrahydroisoquinolines. Alternatively, diazomethane or trimethylsilyldiazomethane was used to synthesize 3-benzazepine derivatives via ring expansion.


 Please wait while we load your content...
Please wait while we load your content...