Recent advances in metal directed C–H amidation/amination using sulfonyl azides and phosphoryl azides
Abstract
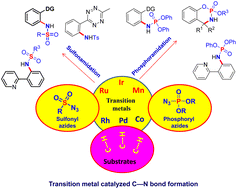
Transition metal-catalyzed C–N bond formation reactions have gained popularity as a method for selectively transforming common C–H bonds into N-functionalized molecules. This approach is particularly useful for synthesizing aminated molecules, which require aminating reagents and amidated building blocks. Over the past two decades, significant advancements have been achieved in transition-metal-catalyzed C–H functionalization, with organic azides emerging as promising amino sources and internal oxidants. This review focuses on recent developments in utilizing sulfonyl and phosphoryl azides as building blocks for directed intra- and intermolecular C–H functionalization reactions. Specifically, it discusses methods for synthesizing sulfonamidates and phosphoramidates using sulfonyl and phosphoryl azides, respectively. The article highlights the potential of C–H functionalization reactions with organic azides for efficiently and sustainably synthesizing N-functionalized molecules, providing valuable insights into the latest advancements in this field.


 Please wait while we load your content...
Please wait while we load your content...