Facile synthesis of sulfinate esters from aryl iodides via direct oxidation of thioesters†
Abstract
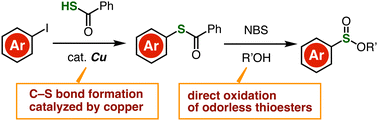
A practical method to synthesize sulfinate esters from aryl iodides is disclosed. Direct oxidation of thioesters prepared by copper-catalyzed C–S formation of aryl iodides realized the efficient synthesis of sulfinate esters. Due to the good accessibility of aryl iodides, a wide variety of sulfinate esters were prepared from easily available starting materials such as carboxylic acids and anilines.


 Please wait while we load your content...
Please wait while we load your content...