Facile access to S-methyl dithiocarbamates with sulfonium or sulfoxonium iodide as a methylation reagent†
Abstract
Efficient access to S-methyl dithiocarbamates was achieved with sulfonium or sulfoxonium iodide as a methylation reagent. This method is reliable for the synthesis of dithiocarbamates from primary or secondary amines, with sulfoxonium iodide demonstrating more robust methylation capability than sulfonium iodide. Moreover, it also enables facile access to S-trideuteromethyl dithiocarbamates via sulfoxonium metathesis between sulfoxonium iodide and DMSO-d6 with high yields.
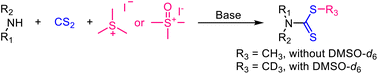


 Please wait while we load your content...
Please wait while we load your content...