NbCl5-catalyzed sulfa-Michael addition for constructing quaternary centers in enones†
Abstract
A novel NbCl5-catalyzed sulfa-conjugate addition has been developed to construct quaternary centers in various enones. This new method enables a range of functionalized thiols to access different β-sulfido carbonyl compounds bearing a quaternary center. 27 novel β-sulfido ketones have been obtained with moderate to excellent yields. The preparative scale reactions also proceed well, showing no decrease in yield. We further studied the mechanism by DFT calculations. This methodology is significant in sulfur chemistry, especially in sulfa-conjugate addition, giving a new pathway to add thiols to tri-substituted enones.
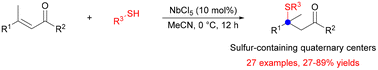


 Please wait while we load your content...
Please wait while we load your content...