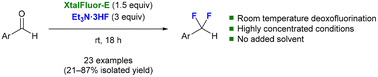
Room temperature deoxofluorination of aromatic aldehydes with XtalFluor-E under highly concentrated conditions†
Abstract
The synthesis of difluoromethyl-containing compounds exploiting the deoxofluorination reaction of aromatic aldehydes using XtalFluor-E is described. This transformation occurs at room temperature under highly concentrated conditions, i.e., with no added solvent. A wide range of difluoromethyl-containing compounds was obtained in 21 to 87% isolated yields.


 Please wait while we load your content...
Please wait while we load your content...