Synthesis and characterization of enantiopure chiral NH2/SO palladium complexes†
Abstract
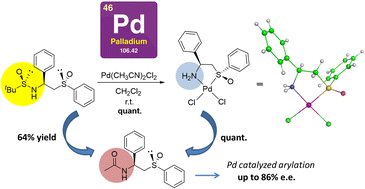
A series of enantiopure chiral NH2/SO palladium complexes have been synthesised with high yields by treating the corresponding tert-butylsulfinamide/sulfoxide derivatives with Pd(CH3CN)2Cl2. The enantiopure chiral ligands were prepared by stereoselective addition of tert-butyl or phenyl methylsulfinyl carbanions to different tert-butylsulfinylimines. In all cases, coordination occurs with concomitant desulfinylation. X-ray studies of the Pd complexes showed a higher trans influence of the phenylsulfinyl group in comparison to that of the tert-butylsulfinyl group. Furthermore, we have obtained and characterised two possible palladium amine/sulfonyl complexes, epimers at sulfur, resulting from N-desulfinylation and coordination of palladium with both oxygens of the prochiral sulfonyl group. The catalytic activity and enantioselectivity of the new Pd(II) complexes of acetylated amine/tert-butyl- and phenylsulfoxides in the carboxylated cyclopropanes arylation reaction has been studied, obtaining the best results with the phenylsulfoxide ligand 25(SC,SS) that produced the final arylated product in a 93 :7 enantiomeric ratio.


 Please wait while we load your content...
Please wait while we load your content...