Accessing spiropiperidines from dihydropyridones through tandem triflation–allylation and ring-closing metathesis (RCM)†
Abstract
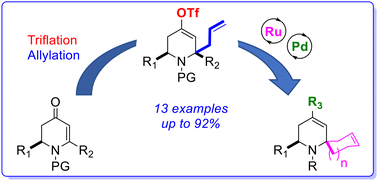
A novel approach to build 2-spiropiperidine moieties starting from dihydropyridones was developed. The triflic anhydride-promoted conjugate addition of allyltributylstannane onto dihydropyridones allowed for the formation of gem bis-alkenyl intermediates that were converted to the corresponding spirocarbocycles with excellent yields via ring closing metathesis. The vinyl triflate group generated on these 2-spiro-dihydropyridine intermediates could be successfully used as a chemical expansion vector for further transformations namely Pd-catalyzed cross-coupling reactions.


 Please wait while we load your content...
Please wait while we load your content...