Regio- and stereocontrolled synthesis of borylated E-enynes, Z-enediynes and derivatives from alkenyl-1,2-bis-(boronates)†
Abstract
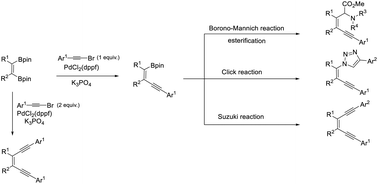
An efficient stereo-controlled synthesis of enyne and enediyne derivatives, via sequential Suzuki–Miyaura coupling reactions from easily prepared 1-alkene-1,2-diboronic esters and alkynyl bromides, is reported. The resulting enyne boronic esters were subjected to Borono–Mannich and Suzuki coupling reactions independently to obtain α,β-unsaturated aminoester and tri-substituted olefin derivatives, respectively. Additionally, divergent syntheses of triazole and cyclopropylboronate derivatives are also reported.


 Please wait while we load your content...
Please wait while we load your content...