Aryl sulfonyl fluoride synthesis via palladium-catalyzed fluorosulfonylation of aryl thianthrenium salts†
Abstract
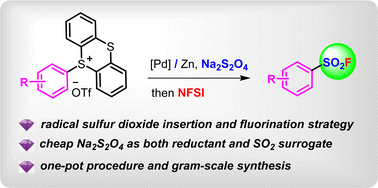
We developed an efficient palladium-catalyzed fluorosulfonylation reaction of aryl thianthrenium salts to smoothly prepare various aryl sulfonyl fluorides using cheap Na2S2O4 as a convenient sulfonyl source in combination with N-fluorobenzenesulfonimide (NFSI) as an ideal fluorine source under mild reduction conditions. A one-pot synthesis of aryl sulfonyl fluorides starting from various arenes was established as well without the need for separating aryl thianthrenium salts. The practicality of this protocol was demonstrated by gram-scale synthesis, derivatization reactions, and excellent yields.


 Please wait while we load your content...
Please wait while we load your content...