A single site mutation can induce functional promiscuity in homoserine kinase†
Abstract
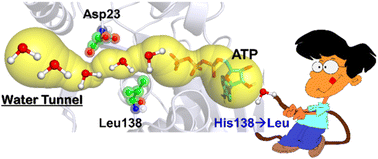
L-Homoserine kinase is crucial in the biosynthesis of L-threonine, L-isoleucine, and L-methionine, where it catalyzes ATP-dependent phosphorylation of L-homoserine (Hse) to yield L-homoserine phosphate as its native activity. However, a single site mutation of H138 → L shows the emergence of ATPase activity as a secondary function. However, a previous mechanistic study proposes direct involvement of ATP and the substrate without any catalytic base; therefore, how the mutation of H138 → L causes the secondary function remains an enigma. Using computational tools herein, we provide new insight into the catalytic mechanism of L-homoserine kinase, showing direct involvement of H138 as a catalytic base. We show that mutation of H138 → L opens a new water channel connecting ATP, which facilitates the ATPase activity and reduces the native activity. The proposed mechanism agrees with the experimental finding that an H138 → L mutation reduces the kinase activity but enhances a promiscuous function, i.e. ATPase activity. Since homoserine kinase catalyzes the biosynthesis of amino acids, we believe that an accurate mechanism could be significant for enzyme engineering to synthesize amino acid analogs.


 Please wait while we load your content...
Please wait while we load your content...