Peptide condensation and hydrolysis mechanisms from a proton-transfer network perspective†
Abstract
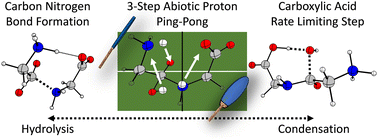
Peptide bond formation is a fundamental organic chemical reaction; however, despite numerous recent reports, the computationally predicted barriers remain contradictory to experimental results. Incompleteness of the molecular mechanism for either the peptide bond formation or the reverse hydrolysis reactions is also highlighted by our lack of understanding of the seemingly equilibrium nature of the reaction that favors dipeptide formation over longer peptide chains under hydrothermal conditions. In this work, we first completed an assessment of levels of theory and evaluated chemical models that range from the neutral glycine condensation reaction in gas phase to explicitly solvated zwitterionic amino acids that are embedded in a polarizable continuum at neutral pH. Ultimately, we identified a six-step ‘ping-pong’ mechanism with the involvement of both zwitterions and neutral species. The carboxylate and amine end-groups of the diglycine intermediates play critical roles in the proton transfer and condensation processes. The experimental condensation barrier of 98 kJ mol−1 was approximated to be 118–129 kJ mol−1 at the MN15/def2TZVPP|SMD(water) level of theory for the rate determining step, when using the most complete model for the solvation environment. Condensed phase free energy correction employed to the rate limiting step reduced the barrier height to 106 kJ mol−1. These results have fundamental implications for understanding enzyme catalyzed peptide bond formation, peptide/protein stability, and “metabolism first” emergence of life scenarios.


 Please wait while we load your content...
Please wait while we load your content...