Synthesis of a fully protected long-chain polyamine subunit of aculeine B using the photoremovable NPEC group†
Abstract
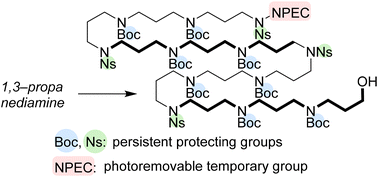
Polyamines are ubiquitously found in nature. In this paper, we disclose our iterative coupling strategy for the synthesis of a structurally defined polymer of 1,3-propanediamine, and the polymer can be used for the synthesis of both the initially proposed structure and the revised structure of protoaculeine B isolated from a marine sponge. We first attempted the synthesis of polyamines using “the Ns strategy” but found that a polyamine with eleven Ns groups has solubility problems. We then examined the versatility of the photoremovable NPEC protecting group in polyamine synthesis. Finally, the synthesis of a suitably protected 12-mer polyamine was achieved employing the NPEC group for the temporary protection of a terminal amino group.


 Please wait while we load your content...
Please wait while we load your content...