Solvent-promoted photochemical carbonylation of benzylic C–H bonds under iron catalysis†
Abstract
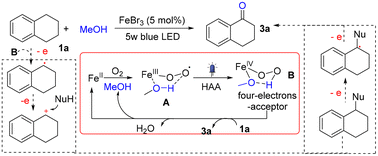
This paper describes the iron-catalyzed photochemical carbonylation of benzylic C–H bonds resulting in the synthesis of various aryl ketones. Using 5 W blue LED irradiation, the reactions proceed smoothly in the presence of 2 mol% of FeBr3 in MeOH at 35 °C. The catalytic system could be extended for the oxidation of silane, thioether, and phosphine into silenol, sulphoxide, and phosphoxide, respectively. A mechanistic study suggests that a hydrogen bond-stabilized iron-hydroperoxo species is the reactive intermediate. It is shown that the reaction proceeds via a four-electron-transfer pathway, and a benzylic cation seems to be the crucial reactive species. The method is applied for the synthesis of pomalyst, haloperidol, melperone, and lenperone.


 Please wait while we load your content...
Please wait while we load your content...