Chemoenzymatic synthesis of (+)-isoagatholactone, (+)-spongian-16-one, and 3-deoxychavalone A via biocatalytic polyene cyclization†
Abstract
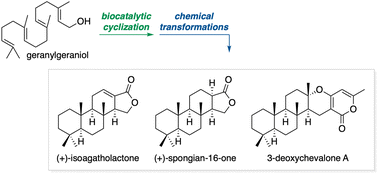
The stereoselective cyclization of geranylgeraniol catalysed by squalene-hopene cyclase (SHC) was investigated. By use of this transformation, spongiane diterpenoids (+)-isoagatholactone and (+)-spongian-16-one, and meroterpenoid 3-deoxychavalone A were synthesized in a concise and redox-economic manner. This work showcases the application of SHC-catalysed cyclization as a key step in terpenoid synthesis.


 Please wait while we load your content...
Please wait while we load your content...