A potent fluorescent transmembrane HCl transporter perturbs cellular pH and promotes cancer cell death†
Abstract
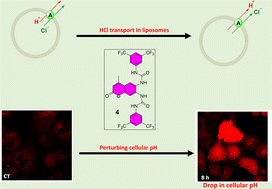
A series of fluorescent coumarin bis-ureas 1–4 have been synthesised, and their anion transport properties studied. The compounds function as highly potent HCl co-transport agents in lipid bilayer membranes. Single crystal X-ray diffraction of compound 1 showed antiparallel stacking of the coumarin rings, stabilised by hydrogen bonds. Binding studies, using 1H-NMR titration, showed moderate chloride binding in DMSO-d6/0.5% with 1 : 1 binding mode (for transporter 1) and 1 : 2 binding mode (host: guest, for transporters 2–4). We examined the cytotoxicity of compounds 1–4 against three cancer cell lines, lung adenocarcinoma (A549), colon adenocarcinoma (SW620) and breast adenocarcinoma (MCF-7). The most lipophilic transporter, 4 showed a cytotoxic effect against all three cancer cell lines. Cellular fluorescence studies showed compound 4 crossed the plasma membrane and localised in the cytoplasm after a short time. Interestingly, compound 4, lacking any lysosome targeting groups, was co-localised with LysoTracker Red at 4 and 8 h in the lysosome. Cellular anion transport of compound 4 was assessed by measuring intracellular pH and showed a decrease in cellular pH, which may be due to the capacity of transporter 4 to co-transport HCl across biological membranes, as evidenced by the liposomal studies.


 Please wait while we load your content...
Please wait while we load your content...