Asymmetric synthesis of spirocyclic isobenzofuranones via a squaramide-catalysed sulfa-Michael desymmetrisation reaction†
Abstract
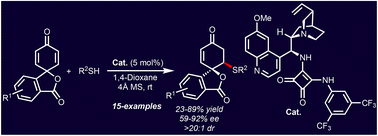
Enantioselective synthesis of spirocyclohexenone isobenzofuranones has been achieved through an organocatalysed sulfa-Michael desymmetrisation reaction. A cinchona-derived squaramide effectively promotes the desymmetrisation of spirocyclic 2,5-cyclohexadienone isobenzofuranones via the controlled addition of various aryl thiols to generate two vicinal stereocenters with perfect diastereoselectivities and up to very good enantioselectivities.
- This article is part of the themed collection: Celebrating 175 years of IIT Roorkee


 Please wait while we load your content...
Please wait while we load your content...