Carbene-controlled regioselectivity in photochemical cascades†
Abstract
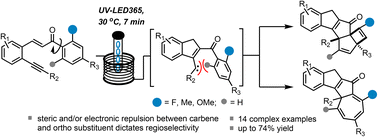
A highly regioselective route to complex carbocyclic scaffolds through a continuous photochemical process is reported. Crucially, we uncovered that ortho substitutents on the right-hand aryl ring are placed away from a transient carbene species which induces the exclusive regioselectivity observed. By varying the non-symmetrically substituted aryl moiety, we demonstrate how the product outcome favors cyclobutenes for electron-poor and neutral substituents and cycloheptatrienes for more electron-rich systems. Additionally, a photochemically induced rearrangement was uncovered for highly electron-rich substrates that ultimately generates complex hydroperoxides. Overall, this facile one-step process is fast and high yielding and demonstrates the power of photochemistry towards the exploration of new chemical space.


 Please wait while we load your content...
Please wait while we load your content...