Synthesis of thiocyanato-containing phenanthrenes and dihydronaphthalenes via Lewis acid-activated tandem electrophilic thiocyanation/carbocyclization of alkynes†
Abstract
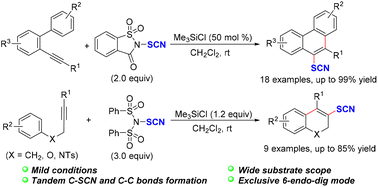
A tandem electrophilic thiocyanation and cyclization of arene-alkynes has been developed under mild conditions, affording thiocyanato-substituted phenanthrenes, dihydronaphthalenes, 2H-chromenes and dihydroquinolines in moderate to excellent yields. This reaction provides an efficient protocol for the construction of C–SCN and C–C bonds in one step. In this transformation, N-thiocyanato reagent serves as a convenient precursor to transfer SCN+ in the presence of trimethylchlorosilane, and the cyclization exhibited exclusive 6-endo-dig selectivity. Finally, a gram scale reaction and further derivatizations highlight the utility of this synthetic strategy.


 Please wait while we load your content...
Please wait while we load your content...